Not like the other fuel cell, diaphragm cells contain a diaphragm, usually made of asbestos fibers, to separate the anode from the cathode. The allows ions to pass through by electrical migration by reduces the diffusion of products. Anodes have usually been made of graphite, cathodes of cast iron. Diaphragms permit the constructions of compact cells of lowered resistance because the electrodes can be placed close together. The diaphragms becoe clogged wit use, as indicated by higher voltage drop and higher hydrostatic pressure on the brine feed. They must be replaced regularly. The diaphragm permits a flow of brine from anode to cathode and thus greatly lessen or prevent side reactions (e.g. sodium hypochlorite formation).
Cells with metal cathodes (titanium coated with rare earth oxides, platinum or noble metals, or oxides) rarely develop clogged diaphragms and operate for 12 to 24 moths without requiring diaphragm replacements. It is expected that diaphragm made of corrosion resistant plastics will increase service life and remove the environmentalist’s objection to any process that may release asbestos fibers into the environment.
A major advantage of the diaphragm cell is that it can dilute (20%), fairly impure brine. Such dilute brines produce dilute sodium hydroxide (typically 11% NaOH with 15% NaCl) contaminated with sodium chloride as a product. Concentration to the usual shipping strength of 50% is required, and this consumes a great deal of energy even when multiple effect evaporators are used. Approximately 2600 kg of water must be evaporated to produce a ton of 50% caustic.
Thursday, February 18, 2010
Friday, February 5, 2010
Primary Cells
Primary cells comprises of two sheet different metal plate or rod or a sheet of metal with carbon rod which placed on the electrolytic bath. The process on the bath will merge different potential between the dipped rods. If using copper rod and zinc rod will result electric potential different between two rod, if connected with wire will flow an electric current from the positive pole (+) to the negative (-). This process will stop sometime because polarization will happen on positive electrode, on this way cell can’t be used again but change with new one. Because of this condition, primary cells can’t be filled.
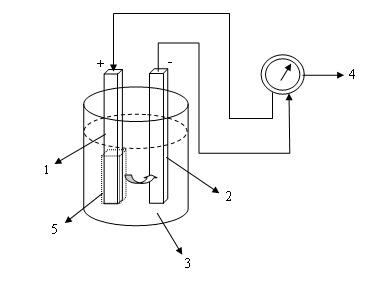
Simple form of Primary Cell: (1) Copper, (2) Zinc, (3) Electrolytic Solution, (4) Ampere meter, (5) Polarization on positive electrode.
Dry Cell also as an example of primary cells form, like on cell battery for electrical appliance that currently used also sample of primary cell form, such as on hand phone, watch, calculator computer and many others. Primary cell just for single use and can’t be filled after used change with new one. Primary cell is very marketable because beside of simple use, also can stand for long time if using the qualified of primary cells.
Simple form of Primary Cell: (1) Copper, (2) Zinc, (3) Electrolytic Solution, (4) Ampere meter, (5) Polarization on positive electrode.
Dry Cell also as an example of primary cells form, like on cell battery for electrical appliance that currently used also sample of primary cell form, such as on hand phone, watch, calculator computer and many others. Primary cell just for single use and can’t be filled after used change with new one. Primary cell is very marketable because beside of simple use, also can stand for long time if using the qualified of primary cells.
Subscribe to:
Posts (Atom)
Popular Post
-
Cheap Price of China Battery China battery can may vary price and normally cheaper than other country producer. China dry battery may ma...
-
Solar Energy in Indonesia still less to use, even Indonesian locate on the equator area. Solar energy can convert into electric energy using...
-
This may often happen to your laptop computer, laptop battery charge drop very fast. If they are charged will fill and full very fast, then...