A Battery is essentially can full of chemicals that produce electron, chemical that produce electron is called as electrochemical reactions. Not every battery can rechargeable, this is because the chemicals inside the battery be activated again so that can function as the original one. Unfortunately this process can't work on and on, sometime the battery will died and can't be charged again.
We we look to the battery itself its contain two terminal or pole, one pole is marked by (+), while the other marked by (-) or negative pole. In large car battery, there are two heavy lead posts that act as the terminals. Electron collect on the negative terminal of the battery. If we connect both terminal by a wire, the electron will flow from the negative to the positive terminal as fast as they can (and battery wear out very quickly). If you do like this can be dangerous especially for large batteries, so it is not recommended to connect of this two pole. If the reaction to fast the battery can be blow away and this very dangerous.
Tips: How to make battery more efficient
Saturday, August 28, 2010
Sunday, August 8, 2010
Make Battery More Efficient
More often you change your old batter, more waste battery will through away. More people know how to treat the battery, make battery more efficient, will make more least waste battery will through away. If you're a recent convert to smartphones, you're probably still discovering all the amazing things that your new BlackBerry, Android phone or iPhone can do. But one thing you most likely found out right away: the more you do, the shorter your phone's battery lasts.
While a standard cellphone's charge can easily go three days or more, many smartphone owners are dismayed to learn that their new mobile toy requires charging every 24 hours, or even more often. It was great that I could use one device — my iPhone — to check my calendar and respond to multiple incoming calls during January's Consumer Electronics Show, but I paid the price when its battery died at 2 p.m.
The answer was not to desperately search for an electrical outlet to recharge the phone (though I've done that) or to consider giving up the phone (done that, too), but rather to figure out a strategy to reduce energy consumption while still having it available for essential tasks. Whether you're using a laptop or a smartphone, the devices can be tweaked to get the most out of its lithium–ion batteries.
While a standard cellphone's charge can easily go three days or more, many smartphone owners are dismayed to learn that their new mobile toy requires charging every 24 hours, or even more often. It was great that I could use one device — my iPhone — to check my calendar and respond to multiple incoming calls during January's Consumer Electronics Show, but I paid the price when its battery died at 2 p.m.
The answer was not to desperately search for an electrical outlet to recharge the phone (though I've done that) or to consider giving up the phone (done that, too), but rather to figure out a strategy to reduce energy consumption while still having it available for essential tasks. Whether you're using a laptop or a smartphone, the devices can be tweaked to get the most out of its lithium–ion batteries.
Friday, May 28, 2010
Type of Battery and Its Waste Classification
Many kind of Battery type and Its Waste Classification can be collected into the table below, every type of battery can result different waste of battery, and the battery waste also can classified into several waste as the hazardous chemical class.
Appearance | Hazardous waste | Can be recycled | |
Alkaline | Alkaline, carbon-zinc, and nickel-cadmium batteries are similar in size and shape, although nickel-cadmium batteries are labeled as such. | If not low-mercury or "green" UI# 7575 D217 & D218 | No |
Carbon Zinc | As above. | No | No |
Lead-acid | Commonly used in cars and motorcycles | Yes UI# 7602 D217 & D218 | Yes |
Lithium | Most large lithium batteries are labeled with the word "lithium" or the initials "LI". Lithium button batteries are smaller and lighter than most types of button batteries and are also unique because they come with only a 3-volt charge. | Yes UI# 7580 D218 | No |
Lithium Ion | Labeled as such or "Li Ion." | No UI# 9111- Non-haz but collected by UIUC D218 | No |
Nickel-cadmium | Labeled as such or "NiCd." | Yes UI# 7578 D217 & D218 | Yes |
Nickel-metal hydride | Labeled as such or "NiMh." | No UI# 9109 Non-haz but collected by UIUC | No |
Mercuric-oxide button | Mercuric-oxide button batteries are easy to distinguish from non-button types of batteries but not from other buttons. | Yes UI# 7634 D217 | Yes |
Secondary Cells (Rechargeable) | Alkaline, carbon-zinc and nickel-cadmium batteries are similar in size and shape, although nickel-cadmium batteries are labeled as such. | No | No |
Silver-oxide button | Silver-oxide button batteries are difficult to distinguish from mercuric-oxide buttons. | Yes UI# 7635 D219 | Yes |
Appearance | Hazardous waste | Can be recycled | |
Small sealed lead-acid flat plates | Most are enclosed in battery packs and are not easily distinguishable | Yes UI# 7602 | Yes |
Zinc-air batteries | Zinc-air are easily identifiable by the holes button in the bottom. | No | No |
Thursday, May 27, 2010
Waste Battery
Every battery models have chemicals and other materials that will rest after used, and then this waster battery can our environment pollute by this rest materials.
Logistic / Purchase Department by the possible means to purchase only low mercury (" green") batteries which are designated with the words, "low mercury", "zero mercury added", a green stripe, or a symbol that signifies environmentally friendly, such as a tree icon.
All users must;
Steps to minimize battery waste:
At the present time there are recycling outlets for lead-acid batteries, mercuric-oxide and silver-oxide button batteries. Find the legal recycling outlets Various consumer and industrial groups are working towards recycling options for other types of batteries.
Battery management
Using the table on reverse, try to identify the type of battery you wish to dispose. If you find that it should be disposed as a hazardous waste, you should request a pickup to a legal disposal company. If it is not considered a hazardous waste, it may be disposed in the trash.
- Waste batteries may be considered hazardous waste because of their corrosive, reactivity, or toxicity.
- Typically Nickel-cadmium batteries exhibit hazardous waste characteristics but low-mercury alkaline and carbon zinc batteries do not.
- Larger mercury batteries would be likely to test as hazardous, and lithium batteries might be considered reactive.
- Lead acid batteries are considered corrosive, as well as toxic. (Gel cell batteries, are a subset of lead acid batteries, and should be treated the same.)
- Button batteries may or may not test as hazardous, depending on their type and size.
Logistic / Purchase Department by the possible means to purchase only low mercury (" green") batteries which are designated with the words, "low mercury", "zero mercury added", a green stripe, or a symbol that signifies environmentally friendly, such as a tree icon.
All users must;
- Substitute rechargeable alkaline batteries for nickel- cadmium to the maximum extent and completely in the end.
- Collect batteries for chemical waste pickup according to the recommendations in the table on the appendix.
- EHS department to ensure proper collection and carry out the legal disposal according to the applicable EHS legislation.
Steps to minimize battery waste:
- Purchase only low mercury ("green") batteries which are designated with the words, "low mercury", "zero mercury added", a green stripe, or a symbol that signifies environmentally friendly, such as a tree icon.
- Substitute rechargeable alkaline batteries for nickel- cadmium.
- Collect batteries for chemical waste pickup according to the recommendations in the table on the reverse side.
At the present time there are recycling outlets for lead-acid batteries, mercuric-oxide and silver-oxide button batteries. Find the legal recycling outlets Various consumer and industrial groups are working towards recycling options for other types of batteries.
Battery management
Using the table on reverse, try to identify the type of battery you wish to dispose. If you find that it should be disposed as a hazardous waste, you should request a pickup to a legal disposal company. If it is not considered a hazardous waste, it may be disposed in the trash.
Thursday, March 25, 2010
Solar Cell Battery
On the current technology improvement, energy can be stored on the battery that is charge for several time using electric current that supply from any source like electric network that should be convert first to DC current and then store on the battery. Electric current source can be supplied from many sources, like from fuel generator, turbine generator, water turbine generator, steam turbine generator, wind generator or from sun light that usually called as Solar Cell.
Currently as the technology improvement, battery can store electric energy longer that before, as I have an emergency lamp, can keep for about 12 hours. Formerly I my emergency lamp just can store electric for about 3 hours. This improvement on battery technology make more efficient on using electric to supply electric to the charger.
On more sophisticated battery is used on automobile as drive motor to move the car, this really needs a perfect battery to store energy. This battery should be able to store energy for longer use, and have bigger power in order to move the car in high speed. If we have perfect battery to store energy we can use sun light to supply energy to the battery. We need solar cell to convert sun light into electric current and then store in the battery. We can use sun light just for 3 to 5 hours a day and can use this energy for a day even longer. So we can very efficient using other nature fuel source to fulfil our energy need on the house, on office, on the road and on small industry like pharmacy.
Solar cell is using material that has effect to exert electric or different potential to other material so that this unite material can build an electric current. This electric current then join together in order can use as electric supply and store on the battery as energy source.
Currently as the technology improvement, battery can store electric energy longer that before, as I have an emergency lamp, can keep for about 12 hours. Formerly I my emergency lamp just can store electric for about 3 hours. This improvement on battery technology make more efficient on using electric to supply electric to the charger.
On more sophisticated battery is used on automobile as drive motor to move the car, this really needs a perfect battery to store energy. This battery should be able to store energy for longer use, and have bigger power in order to move the car in high speed. If we have perfect battery to store energy we can use sun light to supply energy to the battery. We need solar cell to convert sun light into electric current and then store in the battery. We can use sun light just for 3 to 5 hours a day and can use this energy for a day even longer. So we can very efficient using other nature fuel source to fulfil our energy need on the house, on office, on the road and on small industry like pharmacy.
Solar cell is using material that has effect to exert electric or different potential to other material so that this unite material can build an electric current. This electric current then join together in order can use as electric supply and store on the battery as energy source.
Thursday, February 18, 2010
Diaphragm Cells
Not like the other fuel cell, diaphragm cells contain a diaphragm, usually made of asbestos fibers, to separate the anode from the cathode. The allows ions to pass through by electrical migration by reduces the diffusion of products. Anodes have usually been made of graphite, cathodes of cast iron. Diaphragms permit the constructions of compact cells of lowered resistance because the electrodes can be placed close together. The diaphragms becoe clogged wit use, as indicated by higher voltage drop and higher hydrostatic pressure on the brine feed. They must be replaced regularly. The diaphragm permits a flow of brine from anode to cathode and thus greatly lessen or prevent side reactions (e.g. sodium hypochlorite formation).
Cells with metal cathodes (titanium coated with rare earth oxides, platinum or noble metals, or oxides) rarely develop clogged diaphragms and operate for 12 to 24 moths without requiring diaphragm replacements. It is expected that diaphragm made of corrosion resistant plastics will increase service life and remove the environmentalist’s objection to any process that may release asbestos fibers into the environment.
A major advantage of the diaphragm cell is that it can dilute (20%), fairly impure brine. Such dilute brines produce dilute sodium hydroxide (typically 11% NaOH with 15% NaCl) contaminated with sodium chloride as a product. Concentration to the usual shipping strength of 50% is required, and this consumes a great deal of energy even when multiple effect evaporators are used. Approximately 2600 kg of water must be evaporated to produce a ton of 50% caustic.
Cells with metal cathodes (titanium coated with rare earth oxides, platinum or noble metals, or oxides) rarely develop clogged diaphragms and operate for 12 to 24 moths without requiring diaphragm replacements. It is expected that diaphragm made of corrosion resistant plastics will increase service life and remove the environmentalist’s objection to any process that may release asbestos fibers into the environment.
A major advantage of the diaphragm cell is that it can dilute (20%), fairly impure brine. Such dilute brines produce dilute sodium hydroxide (typically 11% NaOH with 15% NaCl) contaminated with sodium chloride as a product. Concentration to the usual shipping strength of 50% is required, and this consumes a great deal of energy even when multiple effect evaporators are used. Approximately 2600 kg of water must be evaporated to produce a ton of 50% caustic.
Friday, February 5, 2010
Primary Cells
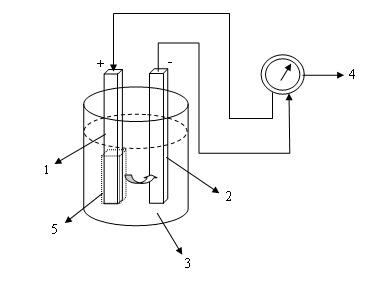
Primary cells comprises of two sheet different metal plate or rod or a sheet of metal with carbon rod which placed on the electrolytic bath. The process on the bath will merge different potential between the dipped rods. If using copper rod and zinc rod will result electric potential different between two rod, if connected with wire will flow an electric current from the positive pole (+) to the negative (-). This process will stop sometime because polarization will happen on positive electrode, on this way cell can’t be used again but change with new one. Because of this condition, primary cells can’t be filled.
Simple form of Primary Cell: (1) Copper, (2) Zinc, (3) Electrolytic Solution, (4) Ampere meter, (5) Polarization on positive electrode.
Dry Cell also as an example of primary cells form, like on cell battery for electrical appliance that currently used also sample of primary cell form, such as on hand phone, watch, calculator computer and many others. Primary cell just for single use and can’t be filled after used change with new one. Primary cell is very marketable because beside of simple use, also can stand for long time if using the qualified of primary cells.
Simple form of Primary Cell: (1) Copper, (2) Zinc, (3) Electrolytic Solution, (4) Ampere meter, (5) Polarization on positive electrode.
Dry Cell also as an example of primary cells form, like on cell battery for electrical appliance that currently used also sample of primary cell form, such as on hand phone, watch, calculator computer and many others. Primary cell just for single use and can’t be filled after used change with new one. Primary cell is very marketable because beside of simple use, also can stand for long time if using the qualified of primary cells.
Subscribe to:
Posts (Atom)
Popular Post
-
Cheap Price of China Battery China battery can may vary price and normally cheaper than other country producer. China dry battery may ma...
-
Solar Energy in Indonesia still less to use, even Indonesian locate on the equator area. Solar energy can convert into electric energy using...
-
This may often happen to your laptop computer, laptop battery charge drop very fast. If they are charged will fill and full very fast, then...