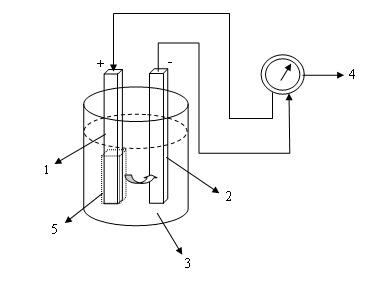
Primary cells comprises of two sheet different metal plate or rod or a sheet of metal with carbon rod which placed on the electrolytic bath. The process on the bath will merge different potential between the dipped rods. If using copper rod and zinc rod will result electric potential different between two rod, if connected with wire will flow an electric current from the positive pole (+) to the negative (-). This process will stop sometime because polarization will happen on positive electrode, on this way cell can’t be used again but change with new one. Because of this condition, primary cells can’t be filled.
Simple form of Primary Cell: (1) Copper, (2) Zinc, (3) Electrolytic Solution, (4) Ampere meter, (5) Polarization on positive electrode.
Dry Cell also as an example of primary cells form, like on cell battery for electrical appliance that currently used also sample of primary cell form, such as on hand phone, watch, calculator computer and many others. Primary cell just for single use and can’t be filled after used change with new one. Primary cell is very marketable because beside of simple use, also can stand for long time if using the qualified of primary cells.
Subscribe to:
Post Comments (Atom)
Popular Post
-
Solar Energy in Indonesia still less to use, even Indonesian locate on the equator area. Solar energy can convert into electric energy using...
-
Currently many battery type on this world, and the most lately battery model is battery recharge. This battery can use several time but jus...
-
More often you change your old batter, more waste battery will through away. More people know how to treat the battery, make battery more e...


No comments:
Post a Comment